 |
Link to the solution
|
|
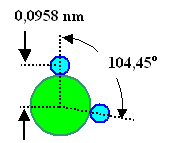
Looking into some standard reference book with numbers, e.g. the "CRC Handbook of Chemistry and Physics", you find that the structure of a water molecule and its dipole moment is | ||||
|
µwater = 1,87 · 10– 18 e.s.u.. | ||||
| 1. How large would the dielectric constant of water be if all water dipoles are completely oriented into the field direction? Or, if you realize right away that this question does'nt make much sense: | |||||
| 1a. How large would the polaization of water be if all water dipoles are completely oriented into the field direction? | |||||
| And what, for gods sake, are e.s.u.? | |||||
|
Link to the solution | |||||
![]() 3.2.4 Orientation Polarization
3.2.4 Orientation Polarization
© H. Föll (Electronic Materials - Script)