|
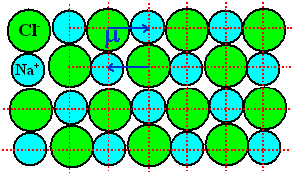
Consider a simple ionic crystal, e.g. NaCl. |
|
 |
The lattice can be considered to consist of Na+ - Cl–
dipoles as shown below. |
| |
|
|
|
|
 |
Each
Na+ - Cl– pair is a natural dipole, no matter how
you pair up two atoms. |
 |
The polarization of a given volume, however, is exactly zero
because for every dipole moment there is a neighboring one with exactly the same magnitude, but opposite sign. |
 |
Note that the dipoles can not rotate; their direction
is fixed. |
| |
| |
|
 |
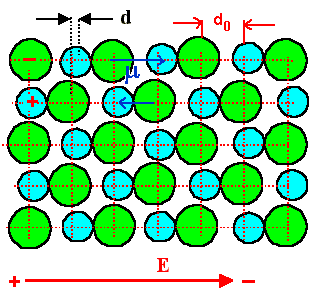
In an electric field, the ions feel forces in opposite directions. For a field
acting as shown, the lattice distorts a little bit (hugely exaggerated in the drawing) |
| |
| |
|
 |
The Na+ ions moved a bit to the right, the Cl– ions to the left.
|
 |
The dipole moments between adjacent NaCl
- pairs in field direction are now different and there is a net dipole moment in
a finite volume now. |
| |
| |
|
 |
From the picture it can be seen that it is sufficient to consider one
dipole in field direction. We have the following situation: |
|
|
|
|
 |
Shown is the situation where the distance between the ions increases
by d; the symmetrical situation, where the distance decreases by d,
is obvious. |
 |
How large is d? That is easy to calculate: |
|
 |
The force F1 increasing the distance is given by |
| |
|
|
 |
With q = net charge of the ion. |
|
 |
The restoring force F2 comes from the binding force, it is given
as the derivative of the binding potential. Assuming a linear relation between binding
force and deviation from the equilibrium distance d0, which is a good approximation for d
<< d0, we can write |
|
|
|
|
 |
With kIP being the "spring constant"
of the bond. kIP can be calculated from the bond structure, it may also be expressed in terms of
other constants that are directly related to the shape of the interatomic potential, e.g. the modulus
of elasticity or Youngs modulus. |
|
 |
If we do that we simply find
|
| |
|
|
 |
With Y =
Youngs Modulus, and d0 = equilibrium distance between atoms. |
 |
From force equilibrium. i.e. F1 – F2
= 0, we immediately obtain the following relations: |
|
 |
Equilibrium distance d |
| |
|
|
 |
Induced dipole moment
m (on top of the existing one)
|
| |
|
|
 |
Polarization P
|
| |
|
 |
Of course, this is only a very rough approximation for an idealized
material and just for the case of increasing the distance. Adding up the various moments - some larger, some smaller - will
introduce a factor 2 or so; but here we only go for the principle. |
 |
For real ionic crystals we also may have to
consider: |
|
 |
More complicated geometries (e.g. CaF2, with ions carrying different amount
of charge). |
|
 |
This example was deliberatly chosen: The dielectric constant of CaF2
is of paramount interest to the semiconductor industry of the 21st century, because CaF2 is pretty
much the only usable material with an index of refraction n (which is
directly tied to the DK via er = n2) that can
be used for making lenses for lithography machines enabling dimensions of about 0,1 mm. |
|
 |
If the field is not parallel to a major axis of the crystal (this is automatically the case
in polycrystals), you have to look at the components of m in the field direction and average
over the ensemble. |
 |
Still, the basic effects is the same and ionic polarization can lead to respectable
dielectric constants er or susceptibilities c.
|
|
 |
Some values are given in the link. |
| |
| |
|
| |
© H. Föll (Electronic Materials - Script)