|
Presently (Oct. 2009) the world at large is in the throes of the financial crisis; before
and after it was (and will be) shaken by the energy crisis. The articles "Terawatt challenge" and "Powering the planet" provide some background
of particular interest to Material Science and Engineering students. |
|
 |
Every budding engineer knows, of course, that automobiles in the near future will run electrically, powered by batteries - at least this is the unavoidable conclusion
if one just reads a newspaper occasionally or listens to news. |
|
 |
Is that true? Are we all going to drive around in electrical cars (or at least serious hybrids) in a few
years that have about the same cost / performance relationship than present gasoline powered cars? This module will provide
a few essentials for pondering this question. |
 |
First, we realize that the energy source in a future car will be a Li ion battery? Why
is that? We will find out by recalling some basic facts about cars and doing a little quiz. |
 |
Before you read on, you should try to figure out the answers yourself by
doing the extremely simple but highly illuminating quizzes (far too simple to be called "exercise") provided in
the links. |
| |
| Quiz 1 |
How much energy is contained in 1 liter of gasoline?
Find out
by using data you know about your car. |
|
|
 |
What you could get is shown in the following table. |
|
|
| Known Property |
SI units | Stupid units |
| Mileage | 10 km/l |
23.5 miles/gallon |
| Range with full tank | 500 km |
311 miles |
| Engine Power P | 100 kW |
135 PS or horse powers |
| Average speed in going 500 km | 120 km/h |
74.6 miles/h |
| Average capacity of engine used | 50 % |
|
| Average efficieny h of engine used |
0,3 or 33% | |
| Time t to cover 500 km |
4.17 h | |
| Energy E consumed = 0,5Pt |
208 kWh | |
Energy EGcontained in 1 l of gasoline
= E/50
l ·h | 12.6 kWh/l |
|
|
 |
Not bad! The "official" number is EG
» 10 kWh/l. |
|
 |
The "»" sign alludes to the (small) differences
between premium, diesel, and so on. Who cares. We are only concerned about orders of magnitude here. |
 |
Is a specific energy of around 10 kWh /l a large
or small number? Find out yourself in the next quiz: |
| |
| Quiz 2 |
How large or small are 10 kWh /l
- in comparison to known energy
(densities). |
|
|
 |
Here are some answers: 1 kWh energy is stored or used up in
- 0,1 l gasoline
- Large (85 Ah) truck battery.
- 0,25 kg dry wood
- 7,3 t H2O in a reservoir with 50 m height difference.
- A weight of 367 t lifted to 1 m.
- 9,5 l brought to a boil (100 oC) from 10oC.
- 100 h of bicycling at 100 W uses up the 10 kWh, moves you about 2.000 km, and works off
about 17 Big Mac's.
|
 |
In other words: The chemical energy contained in gasoline is absolutely
huge compared to typical mechanical energies we can directly relate to! It is, by the way, larger than the energy
stored in 1 kg of a solid high explosive. |
 |
So what is the energy density of a decent battery? Well - it depends. Do you mean energy per liter or per kilogram? There is a big difference from the view point of the user.
|
|
|
|
 |
If you think about electric cars, the weight of the battery
is what you are concerned with. The volume is not all that important. You have plenty of space because you do no longer
need a bulky engine, transmission, drive train and so on. Electric motors are much smaller than comparable gasoline counterparts.
|
|
 |
If you want to power your submarine, it's the the other way around. If the batteries are too light weight,
you have trouble to "sink" it. If you want to power your cell phone, it's volume you are concerned about. If you
want to store huge amounts of energy (e.g. to get through the night if your major energy source are solar cells), weight
and volume are not confining - the price is! |
 |
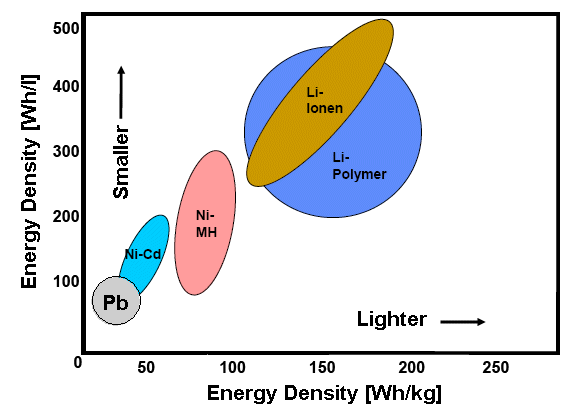
Let's look at both specific energy densities in one diagram |
| |
|
|
 |
It looks like the best we can come up with is at present (Oct. 2009) the Li ion battery with
a specific capacity around 0.5 kWh per liter or 0,15 kWh
per kg. In other words: 1 l of gasoline beats the best battery we have by a factor
of 20 with respect to the specific volume and by more than a factor of 100 with regard to the specific weight. |
 |
OK - that simply means we have to find room for a Li ion battery with a volume of 1000
l or 1m x 1m x 10 cm to run a car. That should be possible in a decent sized car. |
|  |
Unfortunately, if we look at the figure, the weight of such a battery would
be around 3 000 kg or so (and the price would be totally prohibitive). Twice the weight of your old-fashioned standard
car! |
 |
The conclusion is easy: |
| |
If cars are to be powered by batteries in the near future,
we need to improve batteries at at
least by a
factor of 5 or so with respect to the energy density. |
|
 |
As a material scientist you now wonder what exactly determines the energy density of a battery.
For finding out we need to look at the basic working principle of a battery. |
| | |
 |
The energy stored in a battery is chemical energy, i.e. the energy released by a chemical reaction.
We will only consider Li based batteries here, so we have a reaction of
the type: |
| |
|
 |
So take a piece of Li and a "piece" of F, separate it by an electrolyte
that cannot pass electrons but only ions, put it into a box with contacts to the Li and the F - you have a
battery. |
|
 |
It's not so easy, of course. The general principle of forcing the electrons to go through an
outer circuit from one reaction partner to the other one, while the ions move through the electrolyte is certainly correct.
But: |
| |
- How do you keep and contact an (extremely corrosive) gas like F2?
- How do you keep your Li from reacting with the oxygen in the air?
- Will the reaction actually take place? Will a piece of metallic Li in a F2 atmosphere start
to react without being "triggered" somehow (like H2 + O2?
- Can you reverse the reaction by running a current through your battery, i.e. charge it again?
- and so on. and so forth.
|
 |
Making a real battery with "Li ions" is not all
that easy - Point 2 will always a problem, for example, demanding airtight sealing and a lot of security features. |
|
 |
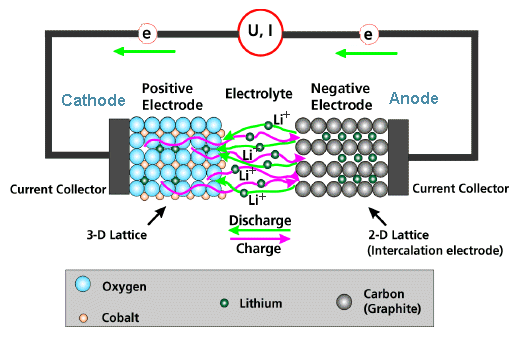
So let's look at the working principle of a Li ion battery
in a very general way. |
| |
|
 |
We have a negative electrode, also called
anode, where Li is present as a "metal". |
| | |
|
|
|
 |
The term negative electrode is clear. The Li atoms
incorporated or intercalated in the electrode material must leave an electron back so
that they can move as Li+ ion through the electrolyte if the battery is discharged and thus supplying
energy to the outside world. |
| |
 |
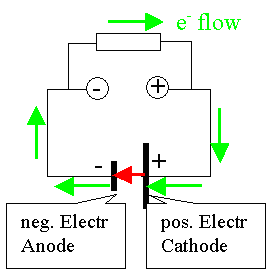
But why is it also called anode? Isn't an anode always the positive
pole towards which the electrons flow? |
| | |
 |
Yeah - but only outside the battery as shown on the diagram. Inside the battery the electrons (or negative charges) must flow from plus
to minus. The battery, after all, is a electron pump that moves the electrons "up" to a high energy
level from which they can "flow down" towards the positive pole |
| |
|
 |
Of course, it's not electrons flowing from plus to minus inside the battery but positively charged Li+
ions in our case. But if you replace the battery by a generator, your electrons flow indeed in the "wrong" direction
inside the generator. |
| | |
 |
The general definition of an anode is therefore: An anode excepts electrons; it provides for a general
oxidation reaction. The polarity is positive seen from an external user and negative as seen from inside a generator. In
the picture you see that the internal electron (or negative charge) current does indeed flow into the anode (red arrows). |
| | |
| |
 |
We have a positive electrode also called cathode (now we know why) where Li reacts to whatever and is incorporated as Li+.
|
|
 |
The chemical reaction to "whatever" - e.g. LiCoPO4 - provides the
energy that drives the whole process. |
 |
Between the electrodes is an electrolyte that
allows Li+ ions to pass but does not conduct electrons. That's why we have a battery. The electrons can
only get from the anode to the cathode by flowing through electron conductors in the external circuit. |
 |
The two electrodes must be intimately connected to some current
collector (a metal like Cu) that conducts the electrical current aut of the battery to the terminals.
|
|
 |
This is not a simple issue! If you want to run a 100 kW motor with a 3 V battery,
you are going to draw 100 000/3 A = 33 333 A which is a lot of current. Even if you switch battery cells in series
to achieve 300 V, you still run 333 A through the current collectors and wires. |
 |
What kind of properties are we asking for concerning the electrode materials? It's a long list;
most important for us at this point are |
|
 |
General Properties:
- Specific capacity or how much Li (in kg) can you incorporate in 1 kg or 1 l of the the electrode
material.
- Electrochemical potential.
- Conductivity; connection to current collector.
- Long term stability; survives how many charge / discharge cycles?
- Hazard potential (does it explode / burn if exposed to air?).
- Price.
- Ecological topics.
|
 |
Negative Electrode/Anode |
|
 |
In principle one could use Li metal. In practice, however, one cannot,
for many reasons. Consider just recharging after all your Li has been used up and is now incorporated in the cathode.
You now have a "hole" on your anode side. How do you get your Li back? |
|  |
We therefore use an anode material that ideally can incorporate a lot of Li
easily, and can release most of that incorporated Li easily. In other words,
it shouldn't take much energy to get it out again. That implies that no strong bonds between the anode material and the
Li must develop. |
|
 |
The standard material at present is graphite.
Li atoms are simply "intercalated" between the hexagonal C-layers. Another, very interesting material
for anodes is Si. We will come back to that. |
 |
Positive Electrode/Cathode Properties |
|  |
In principle same thing as above, except that we must produce a lot of energy
whenever Li+ is incorporated. |
|
 |
What we use are metal oxides (MO) like LiCoO2, LiNiO2,
LiMn2O4, LiFePO4, LiNixCoyMnzO2.
|
 |
The over-all reaction then is like this: |
| | |
| |
| LixC6 | + MO |
Û |
C6 | + LixMO + several eV |
|
|
| | |
 |
All we do, in simple terms, is to "shuttle" Li back and forth
between the two electrodes, gaining energy in one direction (discharge) and using energy (charge) in the other. |
| | |
 |
What determines the capacity of a given Li ion battery? Easy. We first do a quiz to
get some ideas. |
| |
|
 |
What we see is that about 90 g of Li would be enough for a capacity of 1 kWh.
But the diagram above shows that presently we need at least 5.000 g for that. How
can that be? |
|
 |
Well - besides the Li, you need the two electrodes that contain it, an electrolyte,
current collectors, and a housing; not to mention security features. |
|
 |
If we want to improve the gravimetric energy density by at least a factor of 5
as we must, we realize that there is a lot of work out there for enterprising Material Scientists.
What we have to work at are the electrodes (and everything else). The crucial question is: What is the specific capacity
(in mAh/g) of an electrode. In other words: How much Li (in g) can I incorporate into 1 g of
the electrode material. |
|
 |
The reference would be metallic Li, where one obviously can have "1 g Li in 1 g Li"
with a specific capacity of 3 800 mAh/g. It also takes a voltage of 0 V to get Li "out",
i.e. to drive the reaction Li/Li+ because we use that as the zero point of the potential scale. |
|
 |
The relevant voltages are shown for various reactions in the figure. The differnce between the high end
and the low end is what you get as the battery voltage. With a Si/Li anode and a LixCoO2
standard cathode, you should obtain around 3.5 V. |
 |
Let's look what else we have |
| Material | Specific capacity
[mAh/g] |
U vs. Li/Li+
[V] | Comments |
| Graphite | 330 - 370 | 0,1 - 0,6 |
Present standard
anode |
| Li4Ti5O12 | 155 |
1,6 | | | Si |
> 4 000 | 0,1 - 0,5 |
Volume change
factor 4!!! | | Li |
3 800 | 0 | Not practical |
|
 |
|
 |
Now you should be a bit surprised. How can you have more Li in Si
than in Li itself? |
|
 |
Yes - you can. By forming alloys like LiSi, Li12Si7, Li7Si3,
Li13Si4 and Li22Si5. |
 |
So why don't we have Si anodes as a matter of course in our Li ion
batteries? |
|
 |
Because the volume of the Si expands by a factor of 4 when Li in high
concentrations is incorporated. What that means is that your piece of Si
anode will have fractured to fine dust if you load it just once with Li. This is obviously not practical. |
|
 |
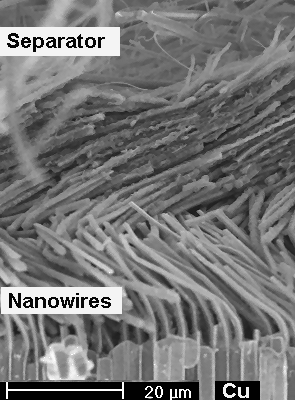
Nevertheless it can be done. The keyword are "nanowires".Overcoming
that problem by using Si nanowires instead of bulk Si is not only a story in itself but brings you to the
heart of "hot" research in materials science and engineering as it is going on right now (Oct. 2009). |
| | | |
© H. Föll (Electronic Materials - Script)