A theoretical introduction to solving numerically ODE is given in section 7.1. Here we will discuss examples of ODE of second order. Our first example is
|
| \begin{equation*} \label{ode_sin_cos} \frac{d^2 y}{dt^2} = -y \end{equation*} | (9.12) |
Translate the above differential equation of second order into a set of differential equations of first order
Check MATLAB HELP for ode45. Here you will find an example very close to the above problem
Copy the corresponding lines into a function and adapt the starting values to find cos(\(x\)) as the solution to the above problem
Adapt the starting values to find sin(\(x\)) as the solution to the above problem
Change the program to find exp(\(x\)) as solution
Change the program to find exp(\(-x\)) as solution
Change the program to find sinh(\(x\)) as solution
Change the program to find cosh(\(x\)) as solution
function [T,Y] = mydglsolv [T,Y] = ode45(@myodefun,[0 2.*pi],[1 0]); plot(T,Y(:,1),’-’,T,Y(:,2),’-.’); function dy = myodefun(t,y) dy = zeros(2,1); dy(1)=y(2); dy(2)=-y(1); end end
Our next example is a reaction scheme specifying three kinetic coefficients \(k_1\), \(k_2\), and \(k_3\) for the following reaction chain
|
| \begin{equation*} {\rm I}_1 \underset{k'}{\overset{k_1}{\rightleftharpoons}} {\rm I}_2 \xrightarrow{k_3} {\rm I}_3 \quad . \label{reaction_scheme} \end{equation*} | (9.13) |
Assuming all reactions to be of first order this scheme translates into a set of differential equations
As starting values we choose \(I_1(0)=1\), \(I_2(0)=0\), and \(I_3(0)=0\). As kinetic coefficients we take \(k_1=1\), \(k_2=0.5\), and \(k_3=0.05\). The implementation in MATLAB is easy:
function [T,Y] = my_chemical_odesolv k1=1; k2=0.5; k3=0.05; [T,Y] = ode45(@myodefun,[0 100],[1 0 0]); plot(T,Y(:,1),’-’,T,Y(:,2),’-.’); function dy = myodefun(t,y) dy = zeros(3,1); dy(1)=-k1.*y(1)+k2.*y(2); dy(2)=+k1.*y(1)-k2.*y(2)-k3.*y(2); dy(3)=+k3.*y(2); end end

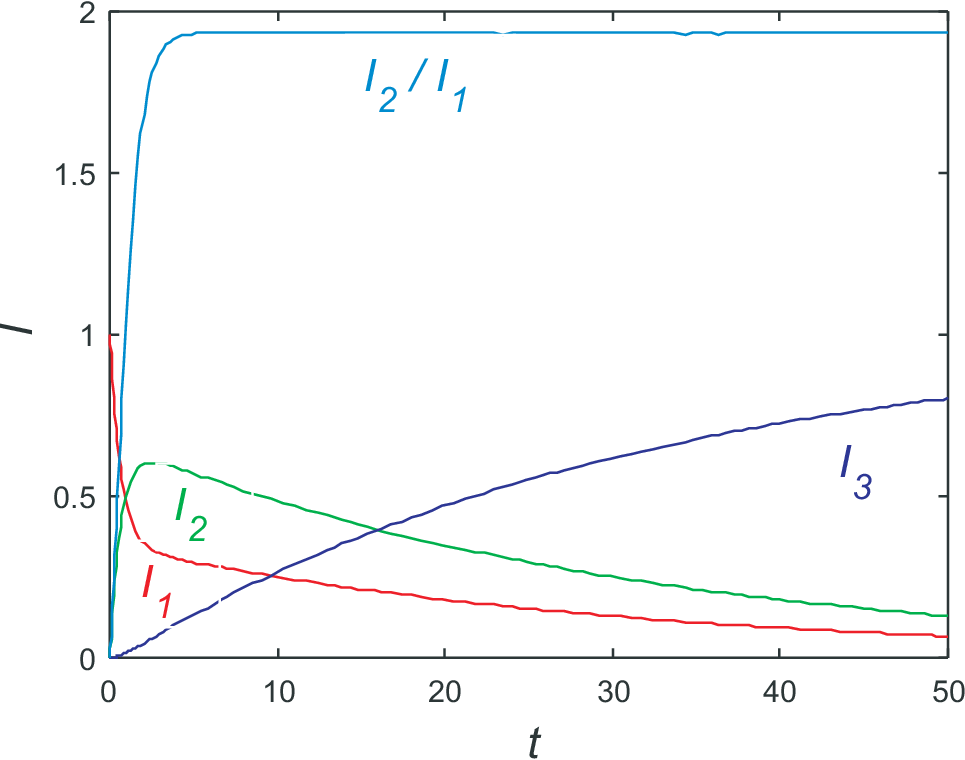
The solution are shown in Fig. 9.3. In what follows we will briefly discuss three concepts often used for discussing transport and kinetics:
rate limiting step
pre- steady state condition
steady state
The rate limiting step is always the slowest process, i.e. that with the smallest
kinetic coefficient, in our case \(k_3\). Somehow counter intuitively not the large kinetic coefficients dominate
the time dependencies, but the slowest process needs the longest time to reach steady state.
Steady
state means that no changes in time exist, i.e. all derivatives on the left hand side of the set of differential
equations are zero. From the third equation we find \(I_3(t\to\infty)=0\). Therefore from the first equation
we find \(I_1(t\to\infty)=0\). From particle conservation we find \(I_3(t\to\infty)=1\). This
are the relations visualized in Fig. 9.3;
additionally the ration of \(I_2/I_1\) is shown. Obviously this ratio reaches a constant value long before
steady state is reached. This effect is called pre-steady state condition and is implied by the large kinetic coefficients
\(k_1\) and \(k_2\). Taking for the moment \(k_3=0\) the (pre-) steady state condition
is reached for \(dI_1/dt = dI_2/dt=0\), i.e. \(k_1/I_1 = k_2/I_2\) or \(I_2/I_1
= k_1/k_2\). This value is very close to the ration found from Fig. 9.3. The deviation can easily be understood from an analysis in linear order in \(k_3\).
We will write
The last equation holds for pre-steady state since in this regime the ration between \(I_2\) and \(I_1\) is constant, i.e. \(\Delta\) is constant.
Including this into the set of differential equations we get
|
| \begin{equation*} \frac{k_1-\Delta}{k_2} = \frac{I_2}{I_1} \approx \frac{dI_2/dt}{dI_1/dt} = - \frac{\Delta-k_3\frac{k_1-\Delta}{k_2}}{\Delta} \end{equation*} | (9.17) |
i.e.
|
| \begin{equation*} k_1\;\Delta-\Delta^2 = -k_2\;\Delta + k_3 (k_1-\Delta) \end{equation*} | (9.18) |
Since we discuss the problem in linear order in \(k_3\), i.e. \(\Delta \propto k_3\), we ignore \(\Delta^2\) (it is tiny!) and find
|
| \begin{equation*} \Delta = k_3\;\frac{k_1}{k_1+k_2+k_3} \end{equation*} | (9.19) |
So at pre-steady state
|
| \begin{equation*} I_2 = \frac{k_1}{k_2}\;\frac{k_1+k_2}{k_1+k_2+k_3}\;I_1 \quad , \end{equation*} | (9.20) |
somewhat smaller than for \(k_3=0\).
For the set of differential
equations we find
Use your program to check for the accuracy of the Eqs. (9.21).
Change your program so that the rate determining step is of second order.
Why does it take much longer before steady state is reached?
Discuss your results in terms of half life time.
Use log plots and log-log plots of your numerical results to identify if the solutions reflect an exponential law (linear order reaction) or a power law (second order reaction).
© J. Carstensen (Comp. Math.)