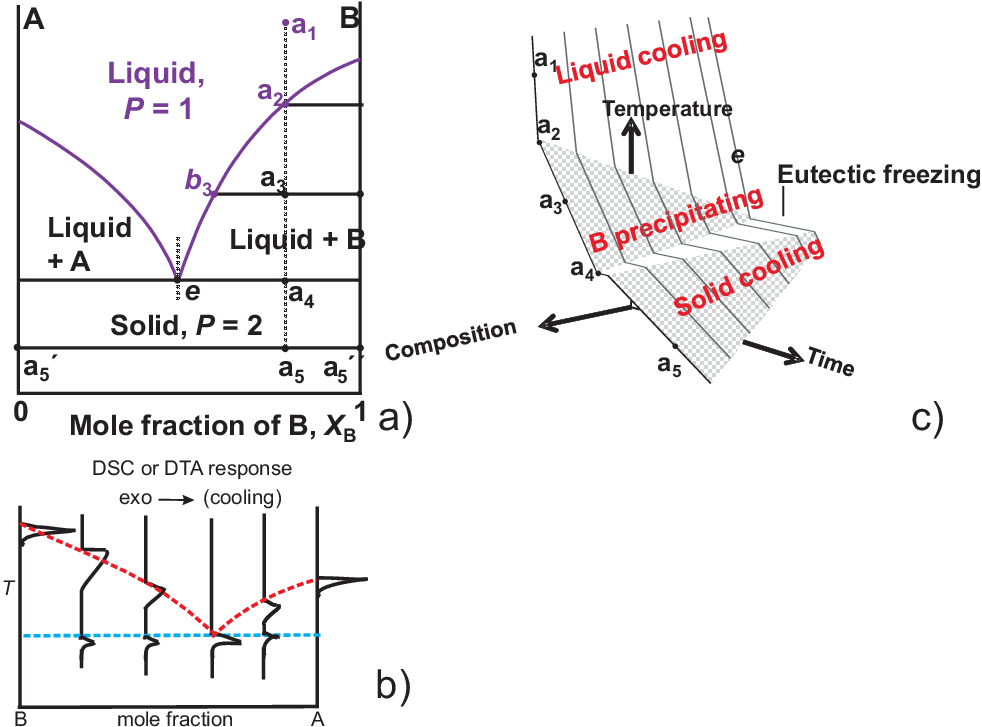
As an example for liquid-solid phase diagrams we will only discuss one example of a eutectic phase diagram as shown in Fig. 2.10 a) and discuss properties along the isopleth \(a\):
\(a_1\) to \(a_2\): single phase melt
\(a_2\) to \(a_3\): Precipitation of pure \(B \rightarrow L\) becomes rich in \(A\)
\(a_3\): further increase of \(A\) in \(L\), solid/liquid phase ratio is close to 1 : 1
\(a_4\): the eutectic composition (\(e\)) of the liquid is reached, further cooling produces a characteristic lamellar micro-structure of pure \(A\) and \(B\). Point \(e\) represents the composition with the lowest melting point, cf. “eutectic” (Greek: “easily melted”).
Experiments for phase diagram determination: Heating or cooling as illustrated in Fig. 2.10 b) and c):
Crystallization of \(B\) releases latent heat slope of cooling curve is reduced
When reaching the eutectic line cooling stops by full crystallization of the sample
Depending on the amount of material with composition \(e\) the plateau could be maintained distinct times. The longest duration occurs for a sample with composition \(e\).

© J. Carstensen (TD Kin II)