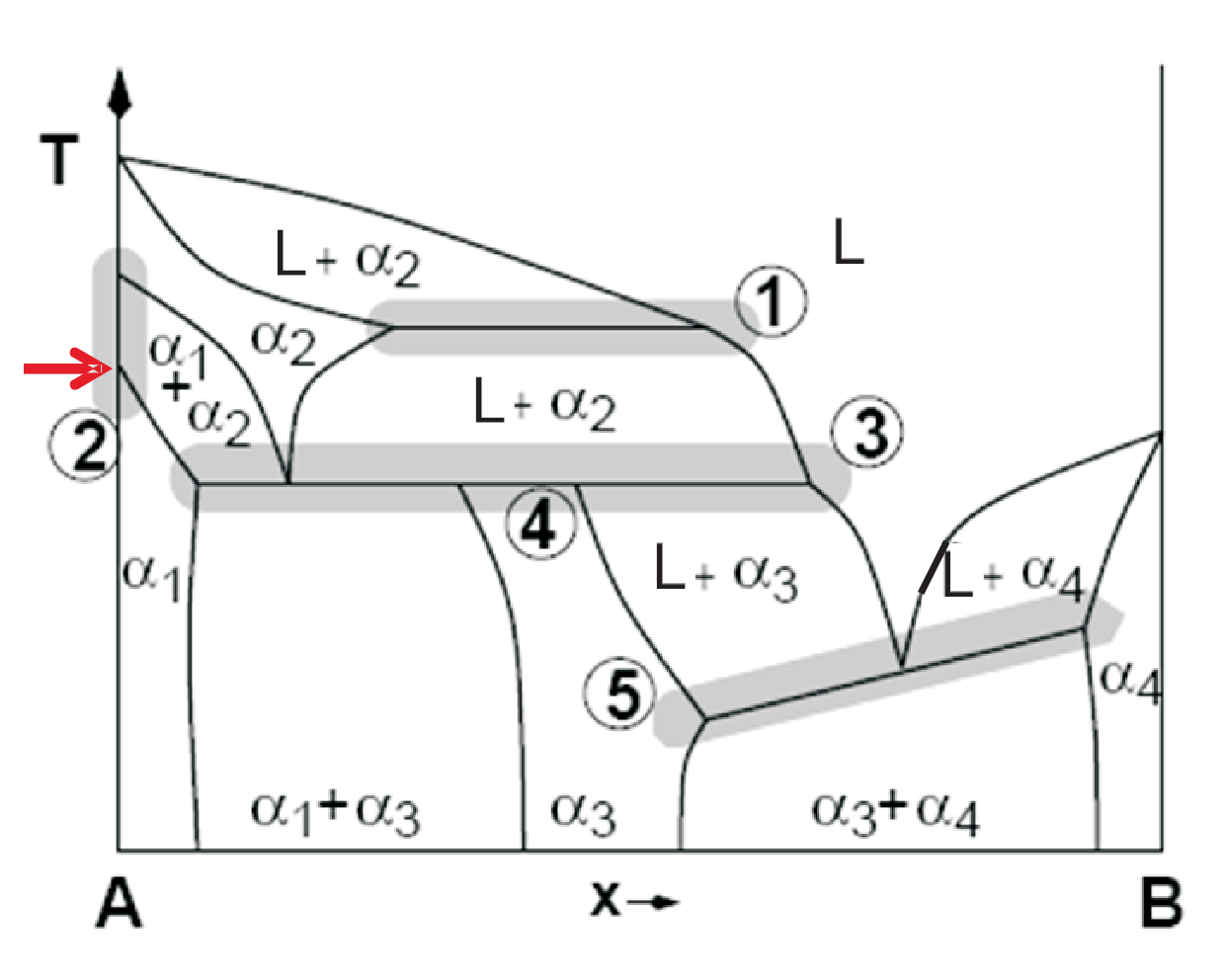
To see how rigorous the Gibbs phase rule is we will discuss the artificial binary phase diagram shown in Fig. 4.1. Since we have a binary phase diagram \(C = 2\). The shown \(x - T\) diagram implies \(p = const.\), i.e. \(P + F = C + 1 = 3\) or \(F = 2 + 1 - P\), thus the maximum number of coexisting phases is 3, i.e. \(F = 0\) which marks a point or horizontal line in the phase diagram. \(F = 1\) marks a sloped line or heterogeneous field. \(F = 2\) marks a homogeneous field.
With this input we now check the lines highlighted in Fig. 4.1.
The horizontal line implies \(F = 0\), however we only have two phases (L and \(\alpha_2\)), i.e. \(P = 2\) but \(P = 3\) needed! No phase boundary can exist between two identical fields (L and \(\alpha_2\)).
This vertical line holds for pure A, i.e. \(C = 1\). Thus having two phases \(F = 0\) is a must, however \(T\) can be varied. Such errors can be induced experimentally by overlooking impurities, i.e. not pure A exists.
A) Neighboring closed areas can not contain more than \(\pm 1\) different phases in contradiction to two neighboring phases \((L + \alpha_2)\):\((\alpha_3)\), B) The max. number of coexisting phases is three in contradiction to two neighboring phases \((L + \alpha_2)\):\((\alpha_1 + \alpha_3)\).
Since \(P = 3\) \((L,\; \alpha_2)\) \(\alpha_3\) we expected \(F = 0\), however \(x\) can be varied.
Three phases (e.g. \(L\), \(\alpha_3\), \(\alpha_4\)) can only coexist at constant \(T\) and \(x\), i.e. \(F = 0\). Thus a horizontal line with \(T = const.\) must exist and no slope is allowed.
![]() Gibbs phase rule including chemical reactions and other restraints
Gibbs phase rule including chemical reactions and other restraints
© J. Carstensen (TD Kin I)