energy is conserved
not force (as was successfully introduced by Newton’s mechanics \( F = m a\)) but energy is the new fundamental concept of physics
which force belongs to the heat energy? (later)
| \(\delta Q\): | heat energy not!! heat stuff: A scientific letter of the 19. century: Blacksmith uses a hammer to form metal; the metal gets warm; the heat stuff flows from the hand into the metal; as a consequence the hand and the head of the blacksmith become cold! |
energy is conserved
not force (as was successfully introduced by Newton’s mechanics \( F = m a\)) but energy is the new fundamental concept of physics
which force belongs to the heat energy? (later)

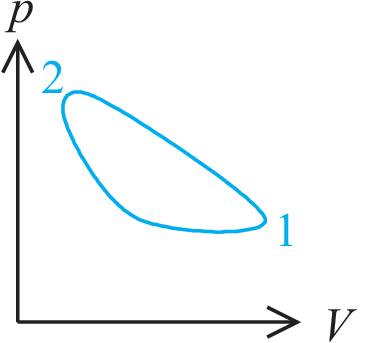
Example:
|
| \begin{equation*} U_{2} - U_{1} = Q - W \end{equation*} | (1.3) |
p.s. \(pV\)-diagrams have been a company secret of James Watt for several
years.
Thus he had a monopoly for good heat transforming machines.
© J. Carstensen (Stat. Meth.)