| |
macropore-formation on p-type-silicon |
| The first experiments | |||
| Propst and Kohl showed 1994 that macropores on p-type-silicon can be etched. They used HF in acetonitril and moderatly doped p-type-wafers. | |||
| Stable macropore-growth on p-type-silicon | |||
| As shown in an paper from Christophersen et al. we showed that large (up to 300 micrometer), small diameters and very stabile macropores in p-type-silicon can be etched. | 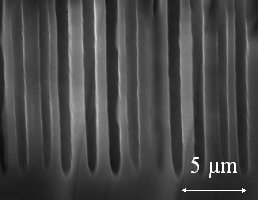 |
||
| Influence of the electrolyte | |||
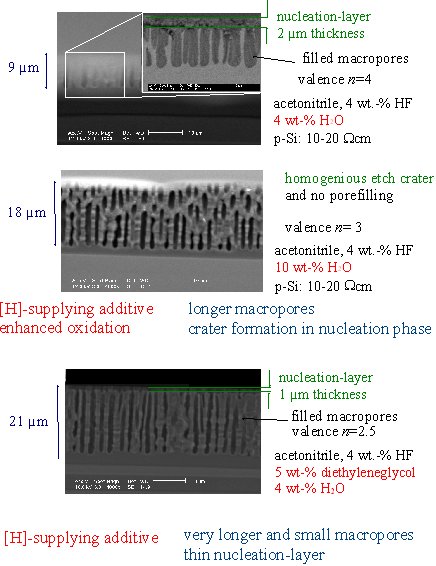
| "Designing of an electrolyte" gives new grades of
freedom for the pore etching system. We studied the influence of additives in
the electrolyte for macropores in p-type silicon. The important parameters are availability of H and oxidizing compontents for the electrochemical reaction. |
 |
||
| In an aqueous HF-containing electrolyte it is possible to etch macropores under special conditions (after V. Lehmann and S. Rönnebeck). The oxide-formation have to be suppressed by the very low etch-potential - the H-termination is still ecfective. | 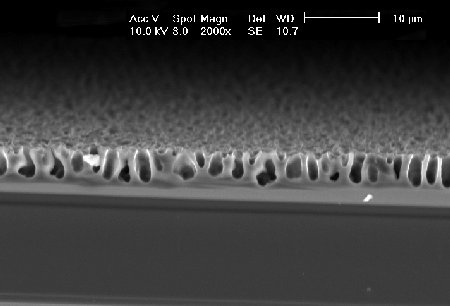 |
||
| Influence of the substrate-orientation | |||
| The macropore-growth is anisotropic in <100> and <113>-direction (the same direction were found in n-type-silicon). The growth direction depends on the surface-orientation. The macropore-walls consists of (111)-facettes - shown by TEM-mircographs. Pore-formation is an anisotropic process. | 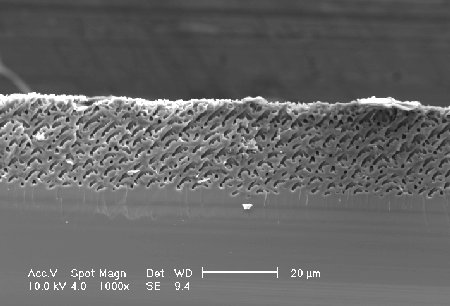 |
||
| for details see: M. Christophersen, J. Carstensen, A. Feuerhake, H. Föll, "Crystal Orientation and Electrolyte dependence fore Macropore Nucleation and stable Growth on p-tye-silicon", Mat. Sci. Eng. B, 69-70, 194 (2000) |
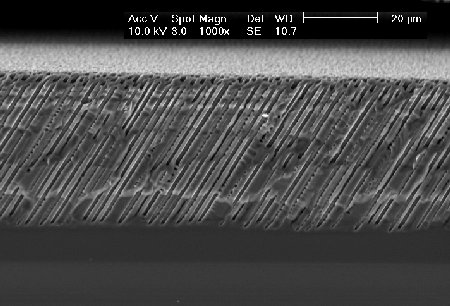 |
||
| These macropores were etched in an aqueous electrolyte. Best macropores in p-type silicon can be obtained in organic electrolytes. | 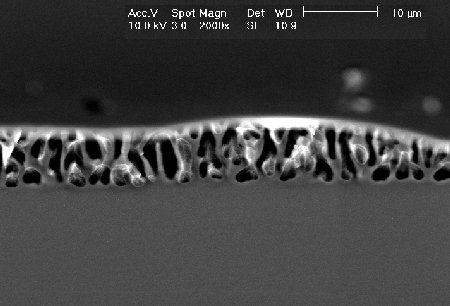 |
||