|
Exercise: Make a Grain Boundary in Salt |
 |
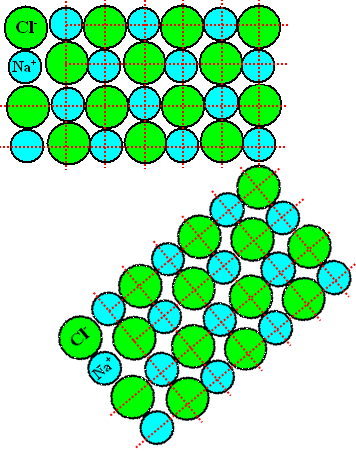
Let's look at a grain boundary in a simple crystal like sodium chloride (NaCl) known as common
salt. Take the two grains shown below that are only rotated with respect to each other and join them, producing a grain
boundary viewed "edge-on" |
| |
 |
The atoms here are charged, they are actually ions. The big green atoms symbolize negatively
charged chlorine, the blue atoms positively charged sodium. Since unlike charges attract each other and like charges oppose
each other, the rules for filling in ions are - Green atoms must only touch blue atoms and vice verse.
- There should be an equal number of blue and grain atoms because their is no net charge.
- Atoms should be closely packed.
|
| |
| |
|
|
|
| | |
|
 |
Can you do it? I can't. That's why there is no link to a solution. |
|
 |
The crystal, however, has no problem to come up with an arrangement of atoms that produces a nice switch-over
from one grain to another one. Not just for the geometry shown here but for any possible geometry.
|
|
 |
To compute the atomic structure, however, is hellishly difficult. |
| | |
© H. Föll (Iron, Steel and Swords script)