|
Again, we have a question here that's a bit more tricky
than it looks |
|
 |
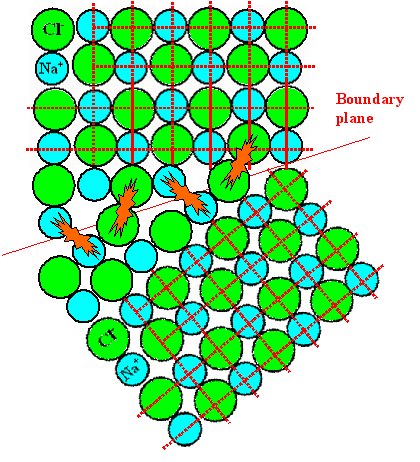
Let's look what we get if we construct a grain boundary along the lines suggested. |
| |
|
|
 |
In this example, some boundary plane was drawn in; then atoms were just added on regular positions to the
top to bottom crystal as long as there was enough space. |
|
 |
The result is not satisfying: We have mans "head-on" situations, were atoms with the same charge
are in intimate contact. This would be a high-energy situation, and that is unlikely. |
 |
The crystal for almost sure will be smarter than us, and arrange its atoms - ions,
to be more precise - in a better way. |
|
 |
Possibly, also atoms a bit away from the grain boundary need to be re-arranged a bit, for that goal. |
 |
What do we learn from that? Two things: |
|
 |
1. The precise atomic structure of grain boundaries in ionic crystal (or any crystals with ionic
components in their bonding) is not easy to predict. In fact, even the precise structure of grain boundaries in simple metal
crystals is far from being simple. |
|
 |
2. Whatever kind of smart structure the crystal will realize, it is quite likely that there will
be some charge imbalance in and around the grain boundary. That means that we also have an imbalance of dipoles - they do
not nearly as nicely cancel each other as the neighboring dipoles in the undisturbed lattice. |
|
 |
And that simply means that grain boundaries - or any kind of interface - will most likely contribute more
than the perfect lattice to the polarization of the material. Of course, only for that part of the "volume" that
it occupies |
| |
|
© H. Föll (Electronic Materials - Script)