|
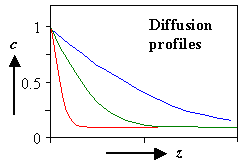
It's easy in principle: You produce and measure a diffusion profile. |
|
|
|
 |
Put whatever is supposed to diffuse on the crystal surface (make sure you cope properly with
the "dirt" or oxide on the surface). | |
|
 |
Let it diffuse at a defined T for a defined time t. |
|
|
 |
Measure the diffusion profile "somehow". |
|
|
 |
Fit to a solution of Fick's law = one data point for D(T). |
|
|
 |
Repeat at different temperatures until you gave enough data points for an (Arrhenius) D(T)
plot. | |
| |
| |
| |
 |
Use isotopes of the material in question for self-diffusion measurements. |
| |
| |
| |
 |
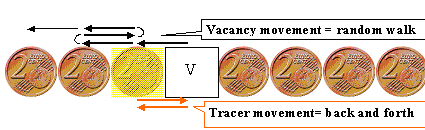
While the intrinsic point defect serving as diffusion vehicle will do a perfectly
random walk, the diffusing atom may not. |
|
|
|
 |
There is a correlation coefficient f linking measured and theoretical diffusion
coefficients. | |
|
| |
|
|
|
| |
|
| |
|
|
 |
The correlation coefficient f is = 0 for 1dim. diffusion, around
1/2 - 2/3 for 2dim. diffusion (e.g. in the base plane of hexagonal lattices) and around 2/3 - 3/4 for
3dim. diffusion. | |
| | |
|
 |
There are many other ways to obtain diffusion data, none fool-proof and all money
and/or time expensive. | |
| | |
| |
© H. Föll (Defects - Script)