|
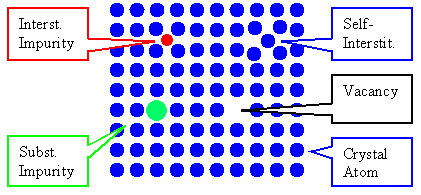
Considering diffusion in crystals we have exactly three basic cases |
|
|
|
 |
1. An interstitial impurity atom diffuses in the crystal=impurity diffusion. |
|
|
 |
2. A substitutional impurity atom diffuses in the crystal=impurity diffusion. |
|
|
 |
3. An atom of the crystal diffuses in the crystal=self-diffusion. |
|
 |
Case 2. and 3. are impossible without diffusion "vehicles",
i.e. vacancies (and on occasion self-interstitials). | |
| |
| |
 |
Diffusion mechanisms are the atomic mechanisms that are capable of moving atoms.
The most important ones are: | |
|
|
 |
Vacancy mechanism. Accounts for most of cases 2. and 3. from above in simple
crystals, | |
|
 |
Direct interstitial mechanisms. Accounts for almost all of case 1. |
|
| |
| |
| |
 |
Some more complex mechanisms exist (and are of prime importance) in Si
(and possibly other semiconductors and somewhat more complex crystals) |
|
Wait and see!
And keep an open mind |
|
|
 |
"Kick-out" mechanism, impurity and self-diffusion via self-interstitials, ... |
|
| |
| |
| |
 |
In any case we need the migration enthalpy Hm and entropy
Sm of the "jumping" entities to obtain the diffusion coefficient D of the
process | |
| Ddir | = |
g · a2 · n
0 · exp | S
k |
· exp – |
Hm
kT |
| DSD | = |
cV · Dv |
| | = |
D* · exp – |
Hm + HF
kT |
|
|
|
 |
Typical values are - like always, it seems - in the 1 eV (better: 0.5 eV - 3 eV)
and 1 k region, respectively. | |
|
 |
Question to ponder: How long does it take for all atoms of crystal to be somewhere else; i.e.
not at the original position? (Exercise 3.2-1) |
|
|
| |
| |
© H. Föll (Defects - Script)