Unfortunately the classification based on eutectoid structure is coarse and little used for practical purposes. Instead, low, medium and high carbon steel are the preferred groupings used.
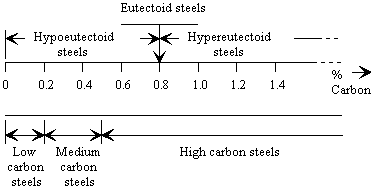 Figure 1 Carbon Steel Classifications |
- Low Carbon Steel (0.06% - 0.25% carbon)
- (0.15% to 0.25% carbon is sometimes called mild steel)
- low cost common construction material
- not considered to be hardenable by heat treatment
- high ductility makes it ideal for press forming (automotive industry, sheets, rods, pipe and wire).
- easily welded, brazed or forged.
- Medium Carbon Steel (0.25% - 0.5% carbon)
- stronger than low carbon steels
- can be further strengthened by heat treatment
- harder than low carbon steels but not hard enough to be used as a cutting tool steel
- weldable but not as easily as low carbon steel
- most supplied as hot rolled and machined to final shape
- High Carbon Steel (0.5% - 1.6% carbon)
- used only where strength and hardness are more important than ductility
- always given a hardening heat treatment
- used for cutting tools
- even at 1% carbon content a drill bit is to difficult to machine in the normalized condition. To allow machining, prolonged heating just below the eutectoid temperature causes the carbide platelets in the pearlite to "ball up". This produces a spherodized structure (machinable). After machining, the drill bit can be reheated to the austenite phase (1500°F / 850°C). Water quenching follows and martensite forms. Martensite is a body centred cubic form of iron that traps carbon atoms that were dissolved in the austenite. The great hardness results from distortions in the lattice caused by the trapped carbons.
Considerable debate exists as to the possibility that true steel production actually occurred in the ancient world. Parr acknowledges the ancients made a case hardened steel but considers this was an accidental by-product of the charcoal next to the bloom. He considers it inappropriate to call the carbon steel alloys made at this time to be the foundation of the steel industry saying this is much like asserting " the baby thrashing the piano next door is making music." [13] Others also fail to consider the manufacture of steel by the ancients to be an intentional industry[14, 15].
Although quality of the steel produced by the ancients must have been poor and inconsistent, they must have strived to achieve a formula for steel. The fact they did not understand what provided steel its desirable properties is no reason to discredit the infant steel industry. Pliny, in his Natural History, Book XXXIV, describes the process of tempering used by Roman blacksmiths. Although his explanations are incorrect, the fact is made that a hardening process was known and used on iron based tools. Pure iron, even very low carbon wrought iron, cannot be hardened. It is only by a knowledgeable process of introducing carbon into iron that the tempering process would have had any effect on tool hardness. Ignorance of the details of tempering continued into medieval times when various processes were attributed to impart the best qualities. Sherby mentions one smith who insisted on quenching the steel in "the urine of a redheaded boy"[16].
Although the exact process was not understood, it was long known that juxtaposition of wrought iron to charcoal increased the hardness of the wrought iron. Two steel making processes were known and practiced in antiquity; the cementation process and the crucible process. The cementation process involved heating wrought iron in contact with a carbon source (usually charcoal) in such a way as to exclude exposure to air. In the crucible process wrought iron bars were melted in crucibles in which charcoal had been placed.
Steel tools made by the cementation process of Roman origin were found in Britain dating to the second century AD[17]. Carbon content varied irregularly throughout from 0% to 1.3%. It was this irregular distribution of carbon that made the cementation process, or "home-made" Roman steel less desirable.
It is suggested by Parr[18] that real production of steel began as early as 500 BC in India. This material was referred to as wootz. By Alexander's time the production of wootz was a well established two step process using the crucible method. Two methods could be used, conversion from a cast iron form or conversion from a wrought iron form.
The first was similar to the simple reduction to wrought iron described above. However, the wootz steel makers used a different version of blast furnace. Iron ore and a carbonaceous material were added together in a crucible, this was called the charge. The charge was placed at the top of the furnace and the blast applied to the bottom[19, 20]. If held at a sufficiently high temperature for a long time the bloom would absorb enough carbon to reduce the melting point of the iron. This would result in the mass being melted and cast iron buttons would form in the crucibles. These would have a high carbon content which would need to be reduced (decarburisation) which was the second step in this process. The cast iron buttons were then reheated and turned in the direct blast flame to a temperature just below their melting point. The buttons could then be heated and welded together by pounding. This process provided a fairly homogenous alloy of steel having 1-1.6% carbon content.
The second method employed seems a more straight forward building process and is suggested by Sherby[21]. After a wrought iron bloom was formed by reduction it was broken into small pieces and placed in a sealed clay crucible with a pre measured amount of charcoal. The crucible was about 7cm in diameter and about 15cm tall. Again the crucible was placed in the blast furnace and heated to about 1200°C until the carbon was absorbed by the iron; thereby reducing the melting point. When the crucible was shaken a sloshing sound was sought to confirm the process had been completed. Slow cooling of the crucible over several days would result in an homogenous alloy of steel with 1.5-2% carbon. During the slow cooling a crystal growth occurs that has a large proportion of iron carbide (Fe3C). Metallurgists identify this white structure on metallographs as cementite. Ancient smiths in the eastern Mediterranean discovered a forging technique that produced an amazing strength and toughness that has only recently been explained[22]. By heating the wootz to a temperature between 600°C and 850°C cementite would not dissolve into the austenite. If it was worked (pounded) at that temperature the cementite crystals would be made smaller and retain the strength of the steel without keeping the brittleness intrinsic with the larger cementite crystals. This metallurgical explanation for the strength and spring properties as well as the swirl colouration of Damascus steels (made by this process since about 330 BC) is a direct contrast to earlier explanations. Friend[23] and Parr[24] are amongst those that explain these steels as a blend of cast iron and wrought iron pounded together. However, the evidence assembled by Sherby and Wadsworth[25] discredits earlier hypotheses advocating the blend of cast and wrought iron.