 |
Where are magnetic dipoles coming from? The classical answer is simple: A magnetic moment
m is generated
whenever a current flows in closed circle. |
|
 |
Of course, we will not mix up the letter m used
for magnetic moments with the m*e , the mass of an electron, which we also need in some magnetic equations. |
|
 |
For a current I flowing in a circle enclosing an area A, m
is defined to be |
| |
|
|
 |
This does not only apply to "regular" current flowing in a wire, but in the extreme
also to a single electron circling around an atom. |
 |
In the context of Bohrs
model for an atom, the magnetic moment of such an electron is easily understood: |
|
 |
The current I carried by one electron orbiting
the nucleus at the distance r with the frequency n = w/2p is
.
|
|
|
|
|
 |
The area A is p
r2, so we have for the magnetic moment morb of the electron |
| |
| morb | = |
e · |
w
2p |
· p r2 |
= |
½ · e · w · r 2 |
|
|
 |
Now the mechanical angular momentum L is given by |
|
|
|
|
 |
With m*e = mass of electron (the *
serves to distinguish the mass m*e from the magnetic moment me of the electron),
and we have a simple relation between the mechanical angular momentum L of an electron (which, if you remember, was the decisive quantity in the Bohr atom model)
and its magnetic moment m. |
|
|
|
|
 |
The minus sign takes into account that mechanical angular
momentum and magnetic moment are antiparallel; as before we note that this is a vector equation
because both m and L are (polar) vectors. |
|
 |
The quantity e/2m*e is called the gyromagnetic
relation or quotient; it should be a fixed constant relating m
and any given L. |
|
 |
However, in real life it often deviates from the value given by the formula. How can that
be? |
|
 |
Well, try to remember: Bohr's model is a mixture of classical physics and quantum physics
and far too simple to account for everything. It is thus small wonder that conclusions
based on this model will not be valid in all situations. |
 |
In proper quantum mechanics (as in Bohr's
semiclassical model) L comes in discrete values only. In particular, the
fundamental assumption of Bohr's model was L = n · 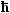 , with n = quantum number = 1,
2, 3, 4, ... , with n = quantum number = 1,
2, 3, 4, ... |
|
 |
It follows that morb
must be quantized, too; it must come in multiples of |
| |
| morb = |
h · e
4p · m*e |
= mBohr = 9.27 · 10–24
Am2 |
|
|
|
 |
This relation defines a fundamental
unit for magnetic dipole moments, it has its own name and is called a Bohr magneton. |
|
 |
It is for magnetism what an elementary charge is for electric effects. |
 |
But electrons orbiting around a nucleus are not the only
source of magnetic moments. |
|
 |
Electrons always have a spin
s,
which, on the level of the Bohr model, can be seen as a built-in angular momentum with the value 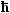 · s.
The spin quantum number s is ½, and this allows two directions of angular momentum and magnetic
moment , always symbolically written as . · s.
The spin quantum number s is ½, and this allows two directions of angular momentum and magnetic
moment , always symbolically written as . |
|
|
|
 |
It is possible, of course, to compute the circular current represented by a charged
ball spinning around its axis if the distribution of charge in the sphere (or on the sphere), is known, and thus to obtain
the magnetic moment of the spinning ball. |
|
 |
Maybe that even helps us to understand the internal structure of the electron, because we
know its magnetic moment and now can try to find out what kind of size and internal charge distribution goes with that value.
Many of the best physicists have tried to do exactly that. |
|
 |
However, as it turns out, whatever assumptions you make about the internal structure of the
electron that will give the right magnetic moment will always get you into deep trouble
with other properties of the electron. There simply is no internal
structure of the electron that will explain its properties! |
|
 |
We thus are forced to simply accept as a fundamental property of
an electron that it always carries a magnetic moment of
|
| |
| me = |
2 · h · e · s
4p
· m*e | = ± mBohr |
|
|
|
 |
The factor 2 is a puzzle of sorts - not only because it
appears at all, but because it is actually = 2.00231928. But pondering this peculiar fact leads straight to quantum
electrodynamics (and several Nobel prizes), so we will not go into this here. |
 |
The total magnetic moment of an atom - still within the
Bohr model - now is given by the (vector)sum of all the "orbital" moments and the "spin"
moments of all electrons in the atom, taking into account all the quantization rules; i.e. the requirement that the angular
momentums L cannot point in arbitrary directions, but only in fixed ones. |
 |
This is were it gets complicated - even in
the context of the simple Bohr model. A bit more to that can be found in the link.
But there are few rules we can easily use: |
|
 |
All completely filled orbitals carry no magnetic moment because for every electron with spin s there is a one with spin
-s, and for every one going around "clockwise", one will circle
"counter clockwise". This means: |
|
 |
Forget the inner orbitals - everything
cancels! |
|
 |
Spins on not completely filled orbitals tend to maximize their contribution;
they will first fill all available energy states with spin up, before they team up and cancel each other with respect to
magnetic momentum. |
|
 |
The chemical environment, i.e. bonds to other atoms, incorporation
into a crystal, etc., may strongly change the magnetic moments of an atom. |
 |
The net effect for a given (isolated) atom is simple. Either it has a magnetic
moment in the order of a Bohr magneton because not all contributions cancel - or it has none. And it is possible, (if not
terribly easy), to calculate what will be the case. A first simple result emerges: Elements with an even
number of electrons have generally no magnetic moment. |
 |
We will not discuss the rules for getting the permanent magnetic moment of a single
atom from the interaction of spin moments and orbital moments, but are going to look at the possible effects if you |
|
 |
bring atoms together to form a solid, or |
|
 |
subject solids to an external magnetic field H |
 |
A categorization will be given in the next paragraph. |
| |
|
© H. Föll (Advanced Materials B, part 1 - script)